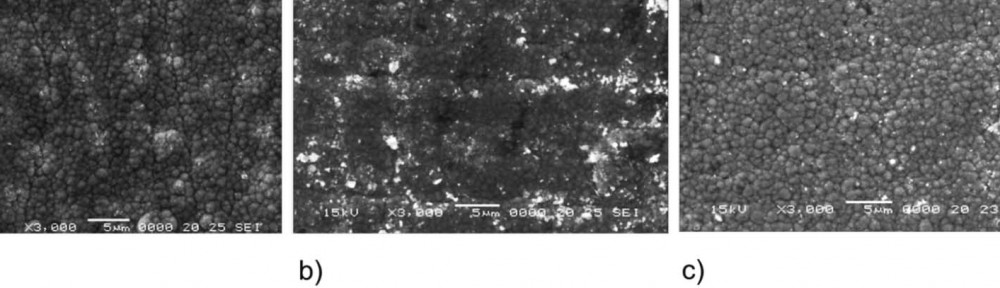
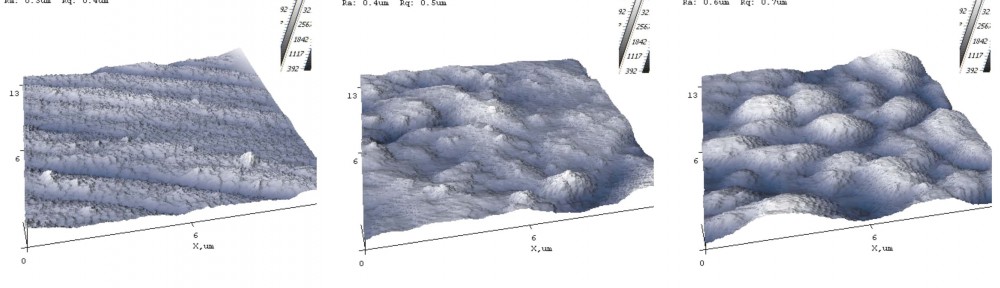
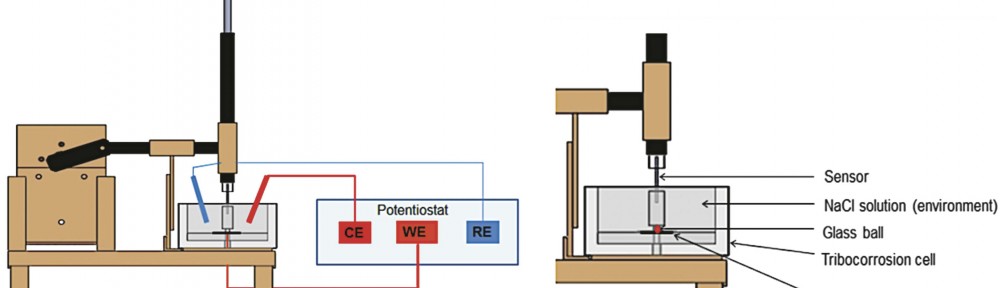
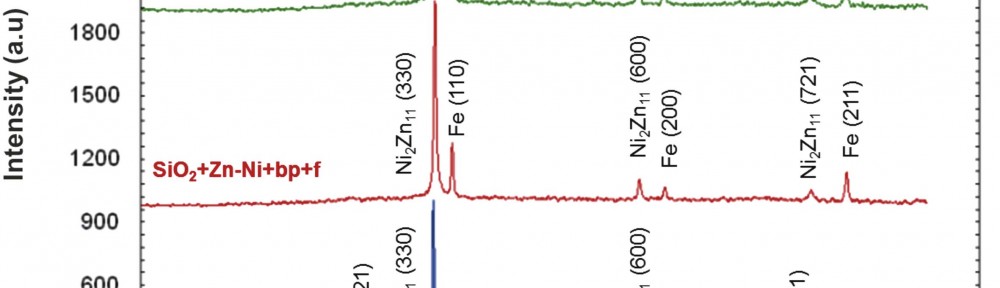
The tribological behavior of an electrodeposited Zn-Ni alloy layer has been investigated to understand the effect of substrate and electroplating methods. The substrates used were: steel, steel/silicon dioxide and steel/silicon dioxide/boron nitride. The tribological behavior was investigated using a ball-on-plate tribometer equipped with an electrochemical cell with 1 % NaCl solution. Open circuit potential measurements, chrono-amperometry (CA) (constant potential electrolysis technique – CPE) measurements and electro-chemical impedance spectroscopy (EIS) measurements were made, before and after a wear test. The coefficient of friction was also measured. The structure and morphology of the electrodeposited layers and the nature of the corrosion products were determined using SEM, XRD and AFM measurements. The Zn-Ni coating electrodeposited using pulse current electrodeposition on steel/silicon dioxide/boron nitride substrate was found to have a higher tribocorrosion resistance compared to the Zn-Ni layers electrodeposited by using pulse current electrodeposition and electrodeposition in a magnetic field on steel/silicon dioxide or by conventionally electrodeposited on steel substrate.
Category Archives: Excerpt from the Journal GALVANOTECHNIK
Corrosion of Hot Dip Galvanized Rebars in Concrete under the Attack of Chlorides and Freeze-Thaw Cycles

This paper presents a mathematical model for the simulation of physical phenomena that appear in the hot dip galvanized rebars corrosion process, compared with the bare steel rebar in concrete, under the attack of chlorides and freeze-thaw cycles. The physical and corresponding mathematical model are presented and the system of governing equations along with initial and boundary conditions were established. The associated numerical model and the numerical methods of discretization and solving the differential equations system on which the mathematical model is based are also presented. The following parameters were considered: concrete humidity, chloride ions content and temperature variation. The time until corrosion initiation, rebar’s diameter reduction, the advance of the corrosion products front and corrosion current variation were determined, according to environment conditions, exposure time and type of rebar used.
Electrodeposition of tin-cobalt alloy as a replacement for decorative chromium

Tin-cobalt alloys were electrodeposited onto nickel-plated brass substrates using a pyrophosphate electrolyte. Deposition conditions were as follows: current density, 0.2-0.3 A/dm2, bath temperature, 45±2°C, pH 8-8.2, ratio of Sn:Co 1.2:1 and current efficiency, approximately 90%. The bath exhibited good throwing power. Deposit morphology was uniform, with cauliflower-like microstructure, mean grain size 125nm. Visual appearance was very similar to electrodeposited chromium with a bluish-white colour. Electrochemical corrosion measurements using Tafel extrapolation and impedance data (as Nyquist plots) showed that Sn-Co alloy and decorative chromium had comparable corrosion resistance with values of 7.77*10-7 A/cm2 and 200 000 Ωcm2 respectively. The results demonstrate that electrodeposited tin-cobalt alloy can be a viable replacement for decorative electroplated chromium.
Oxide Films on Sintered Tantalum for Electrolytic Capacitors
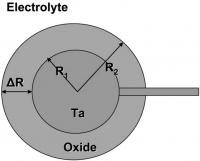
1. Introduction
Electrolytic capacitors are characterized by the phase sequence electronic conductor / dielectric medium / electrolyte where the electronic conductor is a metal (typically Al or Ta) or an oxide (NbO) and the dielectric medium is an insulating oxide film (Al2O3, Ta2O5, SiO2, and Nb2O5). The electrolyte is a viscous or solid. Electrolytes are usually defined as ionic conductors, but for electrolytic capacitors the electronic conductivity is predominant.
Evaluation of Hydrogen Embrittlement Value Due to the Electroplating of Steel Springs

Electroplating is a conventional process for spiral springs coating. One of the major problems in this process is considered to be the undesirable reaction leading to hydrogen embrittlement. Taking into account the application of springs in dynamic conditions, any ductile reduction may cause sudden and quick fracture. Unfortunately, hydrogen embrittlement in steel springs will not come along with any special signs. In addition, evaluation of hydrogen penetration and its consequent embrittlement requires very complex laboratory works, and is time consuming with little repetition due to its being affected by surface conditions as well as steel springs variations.