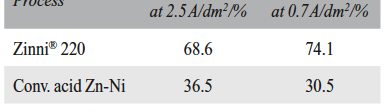
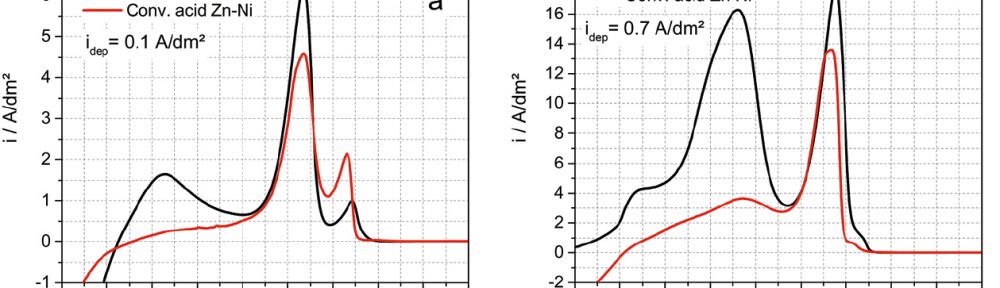
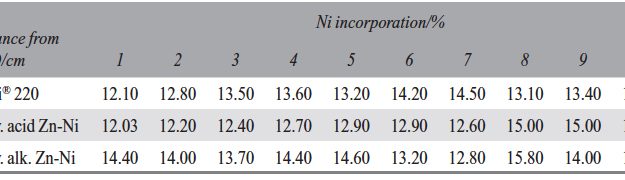
The demand for Zinc Nickel coatings continuously increases in the automotive industry. Especially interesting are zinc nickel alloys with a nickel incorporation of 12–16 %, due to their high corrosion protection as well as superior wear and heat resistance as compared to pure zinc and other zinc alloy coatings.
Despite many advantages of acid Zn-Ni electrolytes there are still some areas of application, like barrel plating or plating of complex-shaped parts, believed to be reserved for alkaline processes. In this paper zinc nickel coatings deposited from ammonium and boric acid-free acid zinc nickel electrolytes, with improved throwing power for rack and barrel applications are investigated. Their corrosion resistance, ductility and hardness will be presented. Moreover, their texture and morphology will be investigated using SEM, XRD and FIB methods. In the end thickness distribution and Ni-incorporation will be presented and compared to alkaline systems.
Category Archives: Excerpt from the Journal GALVANOTECHNIK
New generation of acid Zn-Ni electrolyte for barrel application (Part 1)
The demand for Zinc Nickel coatings continuously increases in the automotive industry. Especially interesting are zinc nickel alloys with a nickel incorporation of 12–16 %, due to their high corrosion protection as well as superior wear and heat resistance as compared to pure zinc and other zinc alloy coatings. Despite many advantages of acid Zn-Ni electrolytes there are still some areas of application, like barrel plating or plating of complex-shaped parts, believed to be reserved for alkaline processes. In this paper zinc nickel coatings deposited from ammonium and boric acid-free acid zinc nickel electrolytes, with improved throwing power for rack and barrel applications are investigated. Their corrosion resistance, ductility and hardness will be presented. Moreover, their texture and morphology will be investigated using SEM, XRD and FIB methods. In the end thickness distribution and Ni-incorporation will be presented and compared to alkaline systems.
Influence of Alloy Composition on Performance of Zinc-Nickel Coatings
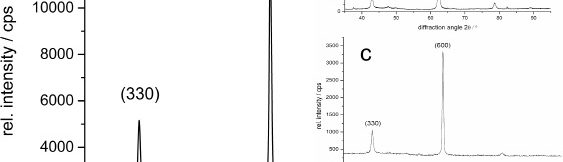
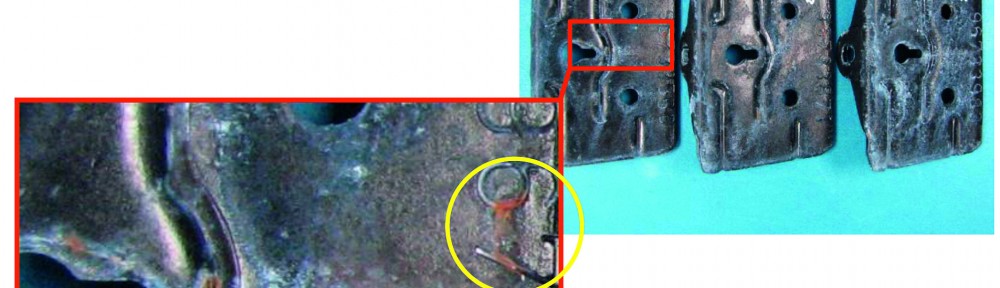
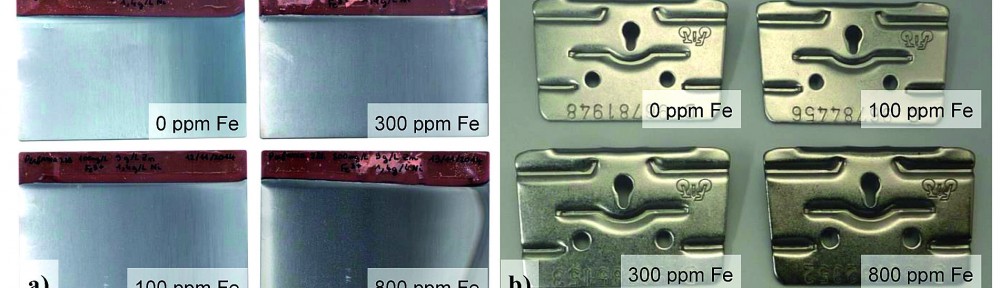
Electrodeposited zinc-nickel coatings are broadly used as sacrificial coatings for steel since many years in the automotive industry and for other high corrosion resistant applications. The best corrosion resistance is obtained with ZnNi deposits having 12–15 % Ni in the alloy. Many studies were performed showing the influence of nickel content in the alloy [1]. In industrial plating electrolytes other metals than Zn and Ni can be present. In alkaline zinc-nickel electrolytes mild steel is usually used as anode material. Depending on electrolyte composition and plating conditions more or less iron can be dissolved by anodic dissolution into the electrolyte. It is well known that the iron is codeposited into the zinc nickel alloy, but the effect on the alloy properties was never systematically investigated. In this study the influence of up to 800 mg/L iron in commercially used alkaline zinc nickel processes is investigated. Up to 8 % iron is amorphously codeposited in the alloy. No new iron containing phases could be detected by X-ray diffraction (XRD). ZnNi g-phases (Ni2Zn11/Ni5Zn21) are still the dominant phases, but plain orientation can be affected by iron codeposition. Corrosion properties are investigated by electrochemical measurements and neutral salt spray test. Whereas no huge difference in the corrosion properties between the bare ZnNi and ZnNiFe coatings was observed, the corrosion resistance with a subsequent trivalent chromium passivate can be drastically improved using iron in the alloy.
Post Treatment of Anodising Layers / Nickel- and Cobalt free Alternatives Working at Ambient Temperatures
NickFor post treating anodising layers on aluminium, typically two different technologies are applied, the hot water sealing at 96-100 °C and the cold sealing using reactive salts to plug the pores of the anodic coating. Both applications show major disadvantages. Whereas the hot water sealing is extremely energy consuming due to the mandatory hot process temperature, the low temperature sealing processes typically apply nickel compounds being harmful to the environment. Nickel salts are toxic and carcinogenetic, having irreversible effects on the human body and health. Furthermore, nickel containing waste waters are difficult to treat, especially when also aluminium is present [1]. New nickel-free technologies have been developed accordingly, enabling a low temperature application yielding in major energy savings. The deposition of antisoluble compounds in the pores of the anodizing layer leads to best stability and corrosion protection, exceeding the performance of hot water sealing. The new process solutions as being non-toxic are less risky to store and to handle, assisting the safety at work. Implementing a new photometrical method for analysing the ingredients, process stability and production quality can be improved [2]. In some cases, the pH-resistance of the anodised surface can be enhanced, extending the application field of anodised aluminium. Moreover, the waste water treatment of the rinses is carried out at pH 9–10, hence, can be done mutually with aluminium containing effluents.
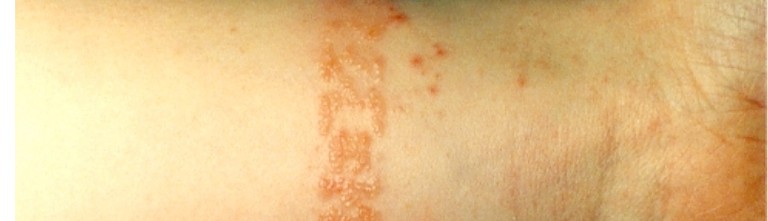
Hybridisation as an efficient joining, electrochemical corrosion study as a need
The composite technology allows the development of structures with a high degree of integration, where the number of elements and auxiliary means for their structural joining are minimised. This can only be achieved by the use of appropriate manufacturing and design processes. Some advantages of such an efficient integration would be the low installation and inspection efforts, shorter cycle times in the production, higher robustness and lower manufacturing costs. Despite this high potential, joining these highly integrated parts is indispensable because of restrictions concerning the components’ complexity, repair requirements as well as material specific limitations [1].