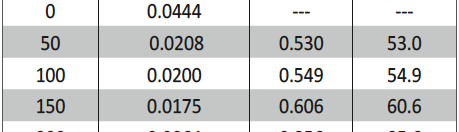
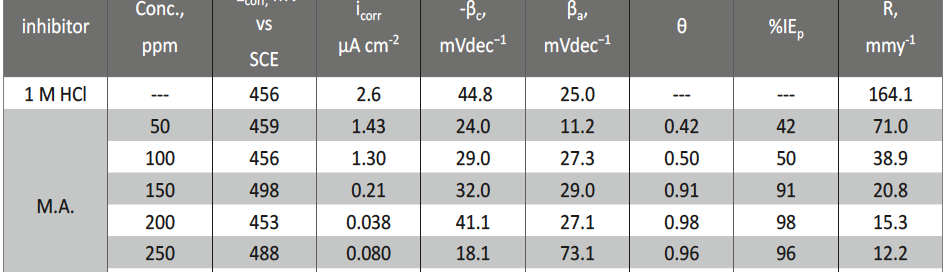
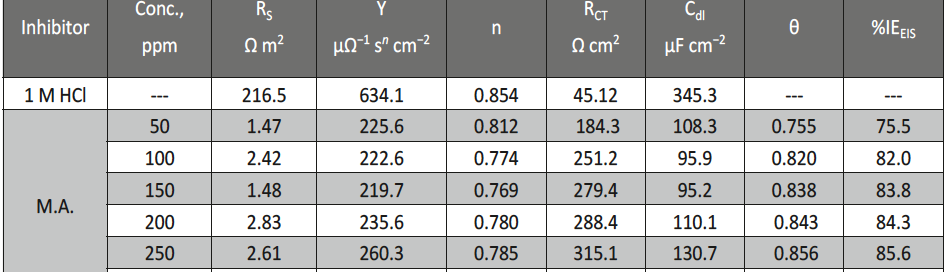
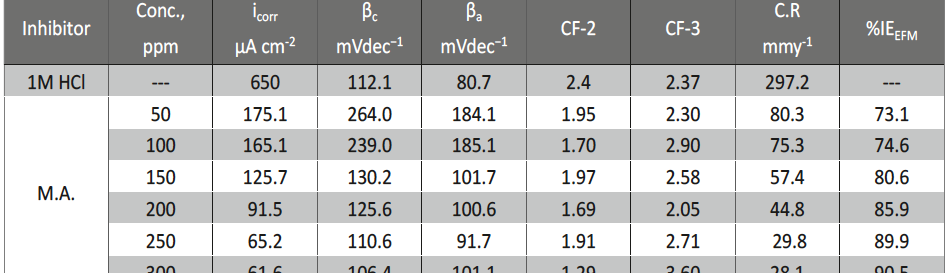
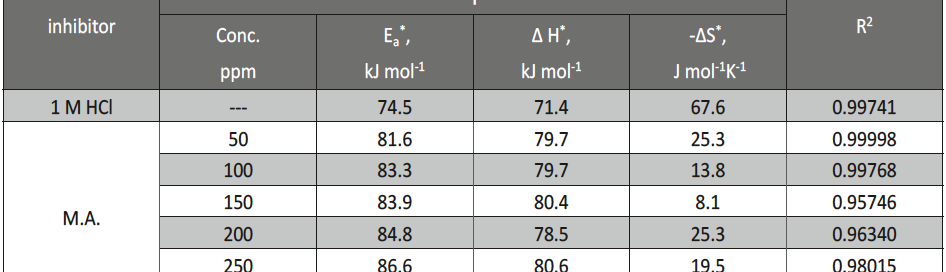
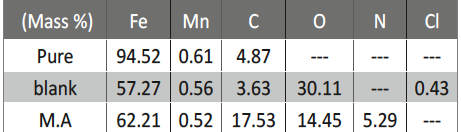
The protection effect of malonic acid on carbon steel corrosion was studied in aerated stagnant 1M HCl solutions at 250C. Measurements were conducted under different experimental conditions using weight loss, Tafel polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. malonic acid was found to be good inhibitor of carbon steel corrosion in1 M HCl. The adsorption of this inhibitor is found to obey the Langmuir adsorption isotherm. The calculated activation energies proposed that the inhibitor molecules being physically adsorbed onto the metal surface. Polarization data revealed that this compound behave as mixed type inhibitor.
Author Archives: Prof. Dr. A. S. Fouda
Adsorption and Corrosion Inhibition Characteristics of Some Thiophene-3-Carbohydrazide Derivatives on Low Carbon Steel in Hydrochloric Acid Solutions
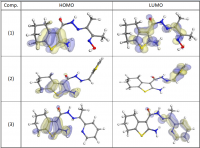
New compounds of corrosion inhibitors namely amino-N’-(3-(hydroxyimino)butan-2-ylidene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (1), amino-N’-(thiophen-2-ylmethylene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (2) and amino-N’-(1-(pyridin-2-yl)ethylidene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (3) were synthesized and its inhibiting action on the corrosion of carbon steel in 1 M hydrochloric acid at 25ºC was investigated by various corrosion monitoring techniques. A Potentiodynamic polarization, AC impedance and electrochemical frequency modulation methods have been used. Potentiodynamic polarization studies showed that these derivatives were mixed type inhibitors. The effect of temperature on the corrosion behavior of carbon steel in 1 M HCl with the addition of these compounds were studied in the temperatures 25 and 45ºC. The adsorption of these inhibitors on carbon steel surface from hydrochloric acid obeyed the Langmuir adsorption isotherm. Quantum chemical method is used to explore the relationship between the inhibitors molecular properties and their inhibition efficiency.
Allium sativum (Garlic) as green corrosion inhibitor for low carbon steel in HCl solutions
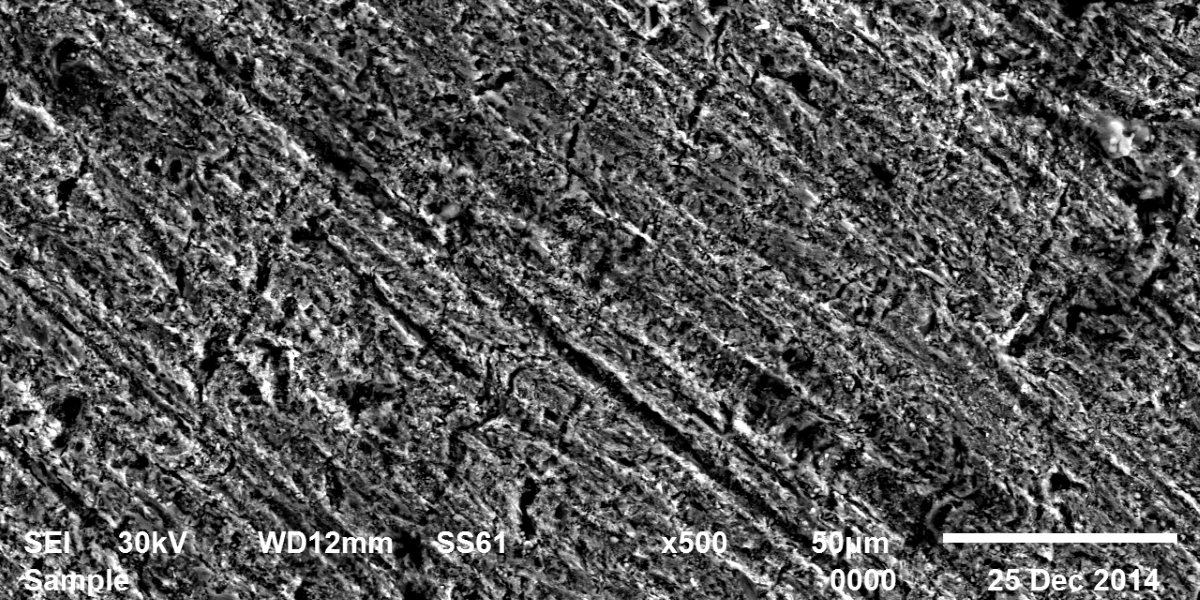
The use of allium sativum (garlic) as a green inhibitor for the corrosion of low carbon steel in 1M HCl has been studied using potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), electrochemical frequency modulation (EFM) techniques and weight loss measurements. Allium sativum has been proved to be good inhibitor. This reduction in the corrosion rate was due to the formation of an external layer formed by S-containing film present in the extract which was adsorbed physically on the metal surface. Allium sativum acted as a mixed type of inhibitor. The inhibition efficiency increases with increasing the inhibitor concentration, but decreases with raising the temperature. The adsorption of allium sativum on the metal surface follows Temkin’s adsorption isotherm. From EFM the causality factors are very close to theoretical values which indicate that the measured data are of good quality. Nyquist plots show a single capacitive loop in uninhibited and inhibited solutions.
The Use of Molybdate, Chromate and Tungstate Anions as Corrosion Inhibitors for Steel in Sulfide Polluted Salt Water
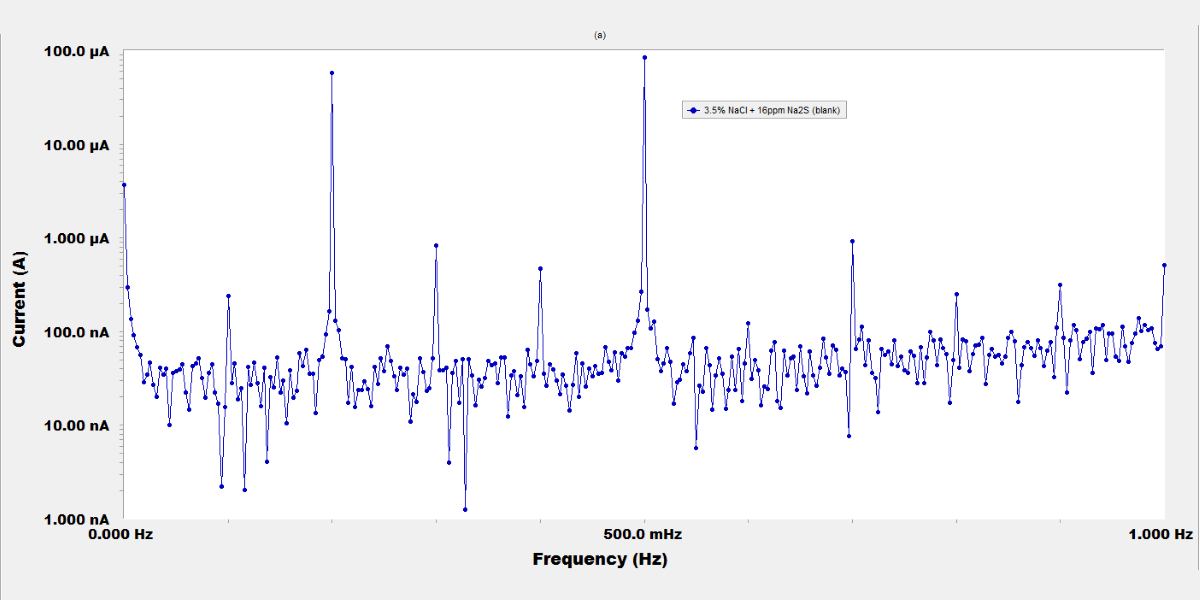
The inhibiting effect of molybdate, chromate, and tungstate salts on the corrosion of steel used in sanitation plants was investigated by potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), and electrochemical frequency modulation (EFM) techniques. The sulfide polluted salt water was simulated by a 3.5 % NaCl and 16 ppm Na2S solution. The results revealed that these inorganic anions are very good inhibitors. Potentiodynamic polarization curves indicated an anodic-type inhibition. The increased concentration of inorganic compounds increases the inhibition; at 250 ppm concentration the inhibition efficiency reached to 95 %, 91.6 %, and 87.5 % for MoO42-, CrO42-, and WO42-, respectively. The adsorption of the inhibitors on the metal surface basically obeys the Langmuir adsorption isotherm equation. Results of EIS measurements suggested that the dissolution of the steel occurs under activation control, and a passive film is probably formed on the metal surface. The electrochemical kinetic parameters calculated from EFM spectra confirm the polarization data.
Coumarin Derivatives as Corrosion Inhibitors for Zinc in HCl Solutions
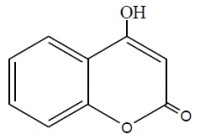
The inhibiting effect of some coumarin derivatives toward the corrosion of Zinc in 0.1M HCl solution was studied using weight loss and galvanostatic polarization techniques. Addition of KI to acidic medium containing the coumarin derivatives increases the inhibition efficiency of the system. The obtained results showed that the inhibition efficiency of these compounds increased by increasing their concentrations and decreased by rising the temperature, so that the adsorption of these compounds is physically adsorbed on the zinc surface. Temkin’s adsorption isotherm fits the experimental data for the studied compounds. Some thermodynamic parameters for the adsorption and activation process were computed. The values of Tafel slopes indicate that these compounds act as a mixed type inhibitors but cathode is more polarized when an external current was applied. The inhibitors are explained in terms of adsorption on the zinc surface. The order of inhibition efficiency are interpreted on the basis of the molecular structure, the subsistent groups and their charge densities of the coumarin derivatives.