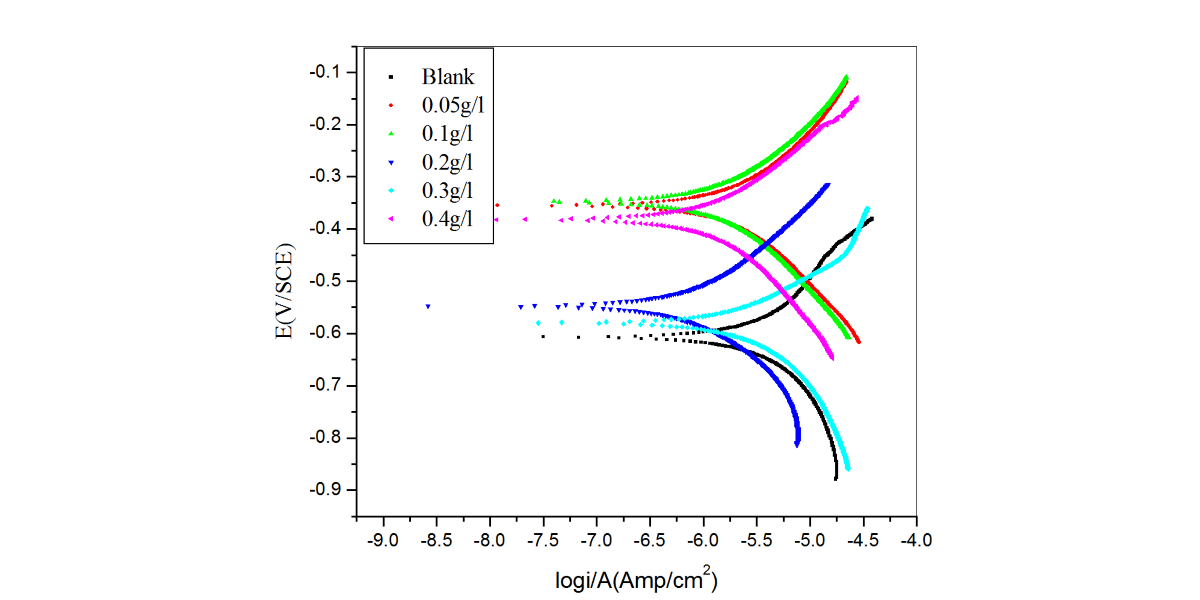
The inhibitive effect of Curry leaves extract (CLE) on the corrosion behavior of aluminum in sulfuric acid (pH = 3) was investigated by potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques in the temperature range of 30 °C to 50 °C. The study was done by varying the concentrations of inhibitor from 0.05 g L−1 to 0.4 g L−1. The surface morphology was studied using scanning electron microscopy (SEM) and Energy-dispersive X-ray spectroscopy (EDX). Inhibition efficiency was found to increase with increase in inhibitor concentration and decrease with increase in temperature. CLE acted as an anodic type inhibitor at lower concentrations of inhibitor and behaved as a mixed type at higher concentrations of inhibitor and underwent both physisorption and chemisorption on the surface of the metal and followed the Langmuir adsorption isotherm.